Acute Kidney Injury Necessitating Outpatient Dialysis
In 2017, the Centers for Medicare and Medicaid Services (CMS) implemented a policy that allowed for outpatient end stage renal disease (ESRD) facilities to provide care to Medicare beneficiaries with acute kidney injury requiring dialysis (AKI-D). Since that time, an increasing number of individuals with AKI-D have received dialysis in the outpatient setting. Fresenius Kidney Care cared for more than 9,000 individuals with AKI-D in a one-year period following implementation of the new policy. Examination of the clinical course and outpatient management of AKI-D is necessary to further the understanding of the AKI-D population and to continue to optimize outcomes in this population.
In the United States, the incidence of AKI-D increased by more than two-fold from 2000 to 2009; in 2009, more than 160,000 hospitalizations included a diagnosis of AKI-D.1 In critically ill patients, the incidence of AKI-D increased nearly four-fold between 1996 and 2010.2 Among patients with AKI-D, the in-hospital mortality rate depends on a number of factors and ranges from 25 to 65 percent.3,4,5 The likelihood of ongoing dialysis following discharge from the hospital varies across studies and is influenced by a number of factors.6,7,8,9
In the United States, the potential treatment settings for sustained AKI-D have changed over time. Prior to 2017, a number of considerations—including the payor, state regulations, outpatient dialysis facility policies, and contractual care arrangements—determined whether AKI-D could be managed in an outpatient dialysis center.10 On January 1, 2017, CMS implemented a policy that allowed for outpatient ESRD facilities to provide care to Medicare beneficiaries with AKI-D.11 This new policy heralded a shift in the care delivery for sustained AKI requiring ongoing dialysis. Given the change in CMS policy and evolving care systems, Fresenius Medical Care North America (FMCNA) undertook an initial examination of the clinical course of AKI-D in the outpatient dialysis facility setting.
AKI-D POPULATION
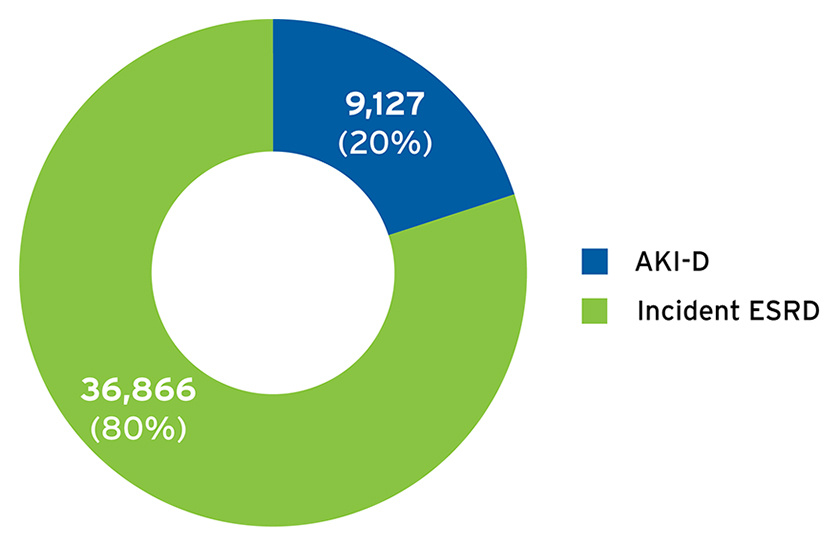
To examine the clinical course of AKI-D, FMCNA included individuals with a first admission to Fresenius Kidney Care centers between April 1, 2017, and March 31, 2018, for treatment of AKI-D. The AKI-D population included 9,127 individuals who initiated care in 2,005 centers (Figure 1). During this one-year time frame, approximately 600 to 900 patients with AKI-D started outpatient dialysis therapy per month, and 20 percent of admissions for the initiation of in-center hemodialysis were for AKI-D (Figure 2).
FIGURE 1 | Geographic distribution of AKI-D cohort
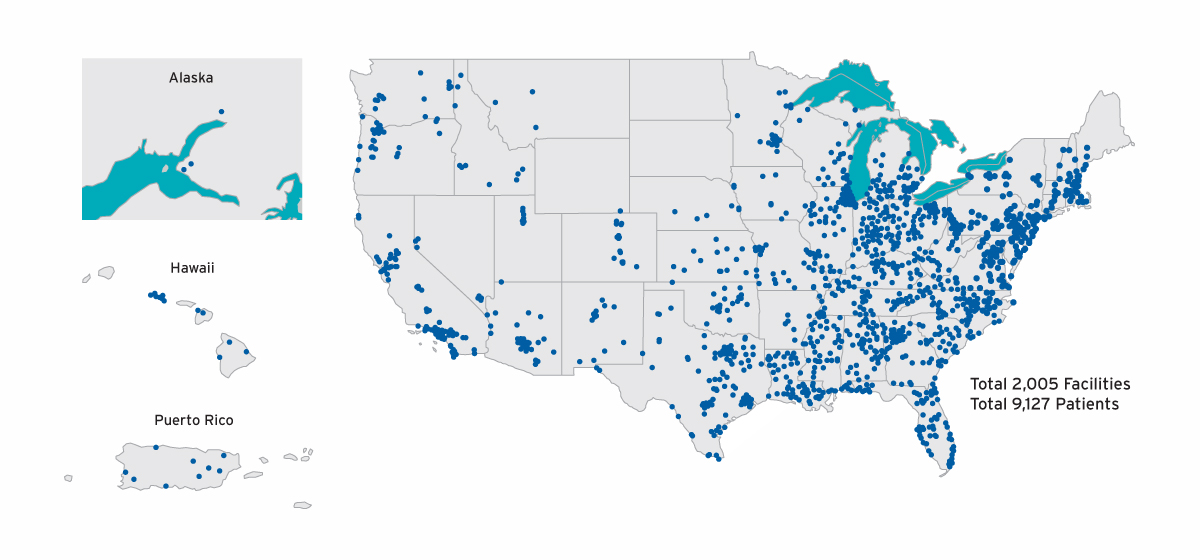
FIGURE 2 | Proportion of patients initiating in-center hemodialysis with AKI-D versus incident ESRD
The AKI-D cohort had a mean age of 63.3 ±14.7 years; 42 percent were female, and 98 percent initiated dialysis with a catheter for vascular access. The initial dialysis prescription was thrice weekly for the vast majority of patients (96 percent), and the mean dialysis treatment time was 223 ±26 minutes.
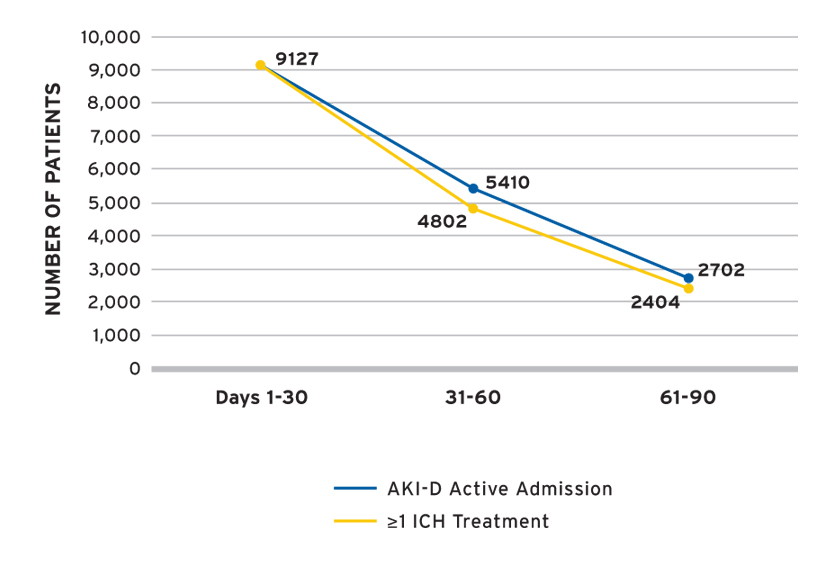
FMCNA examined the first 90 days of AKI-D by 30-day treatment periods and followed the cohort for up to 90 days or until recovery of kidney function, transition to ESRD, withdrawal from dialysis, transfer to a non-Fresenius Kidney Care facility, loss to follow-up, or death. The number of individuals with AKI-D under observation at the beginning of each 30-day treatment period was as follows: 9,127 in days 1-30; 5,410 in days 31-60; and 2,702 in days 61-90. The number of individuals with at least one in-center hemodialysis (ICH) treatment during the treatment period was lower over the latter treatment periods (Figure 3), and accordingly the number of AKI-D observations was lower over time. The availability of data differed by clinical measure, and available data was used to estimate clinical parameters.
FIGURE 3 | Number of individuals with AKI-D by treatment period
DIALYSIS ADEQUACY, BIOCHEMICAL MEASURES, AND VASCULAR ACCESS
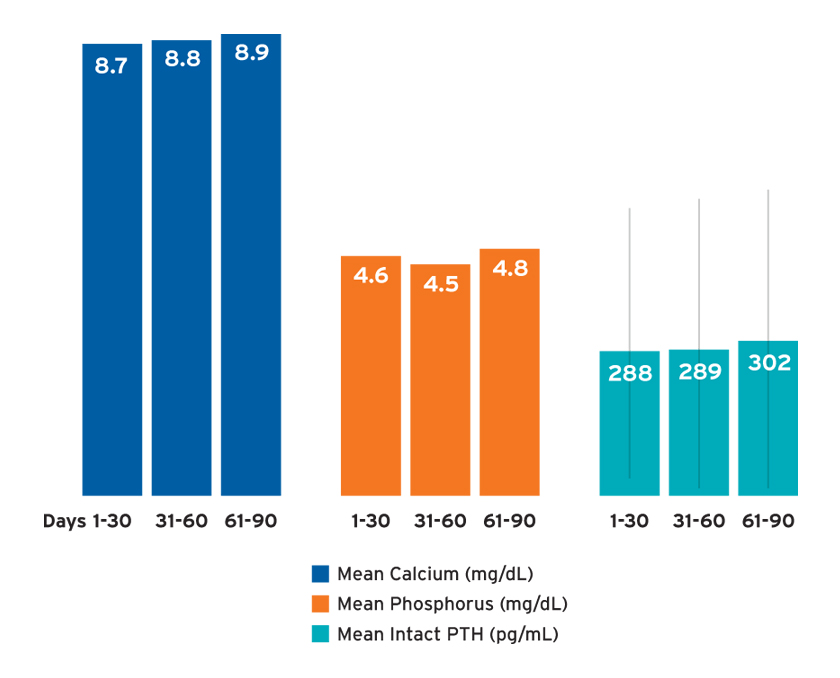
The mean spKt/V (a measure of dialysis adequacy) was 1.5 ±0.4 in days 1-30 and 1.6 ±0.4 in days 31-60 and 61-90. The mean potassium concentration was 4.3 ±0.6 in days 1-30 and 31-60, and 4.4 ±0.6 in days 61-90. The mean 30-day calcium and phosphorus concentrations ranged from 8.7 to 8.9 mg/dL and 4.5 to 4.8 mg/dL, respectively, depending on the time frame of observation (Figure 4). Mean intact parathyroid hormone (iPTH) concentrations ranged from 288 to 302 pg/mL with large standard deviations (Figure 4).
FIGURE 4 | Bone and mineral metabolism labs
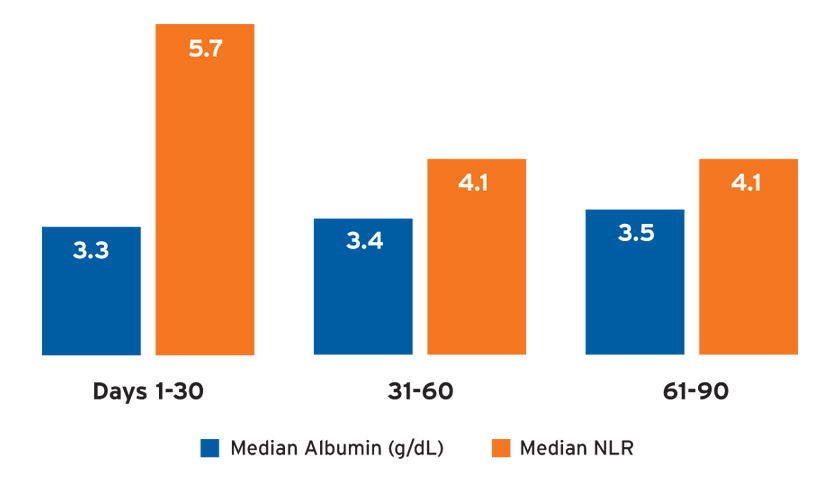
Potential markers of inflammation, including the median albumin and neutrophil-to-lymphocyte ratio (NLR), differed by treatment period (Figure 5). Catheters remained the dominant vascular access in use over time, and 95 percent of the AKI-D population used a catheter for vascular access in days 61-90. Among patients who were using a catheter in the last 30 days of observation, only 4 percent had an arteriovenous fistula or arteriovenous graft present.
FIGURE 5 | Markers of inflammation: albumin and neutrophil-to-lymphocyte ratio
ANEMIA MANAGEMENT
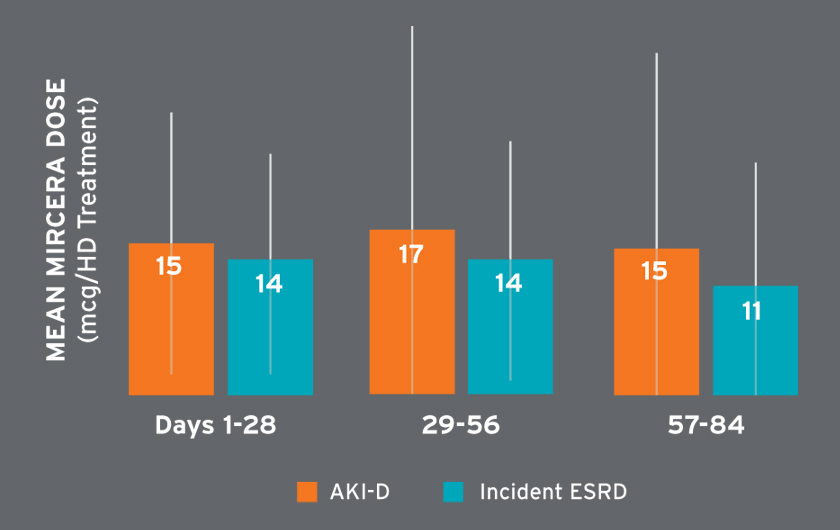
Mean hemoglobin concentrations ranged from 9.3 to 10.5 g/dL and were on average higher in later treatment periods (Figure 6). Among patients receiving Venofer, the mean total dose in the first 30 days was 612 mg followed by mean total doses exceeding 400 mg (Figure 7). The mean ferritin concentration and transferrin saturation among all patients with available laboratory measurements, regardless of IV iron administration, are shown in Figure 7. The percent of AKI-D patients who received an erythropoiesis stimulating agent (ESA) in days 1-28, 29-56, and 57-84 was 53 percent, 80 percent, and 87 percent, respectively. Four-week periods were reported for consistency with ESA dosing frequency. The majority of patients treated with an ESA received Mircera. Mircera dosing ranged from 15 to 17 mcg per hemodialysis treatment (equivalent to approximately 90 to 100 mcg every two weeks); however, doses had a broad distribution as reflected by the large standard deviation (Figure 8). The mean Mircera dose was higher in individuals with AKI-D as compared to incident ESRD patients initiated on in-center hemodialysis (Figure 8).
FIGURE 6 | Hemoglobin
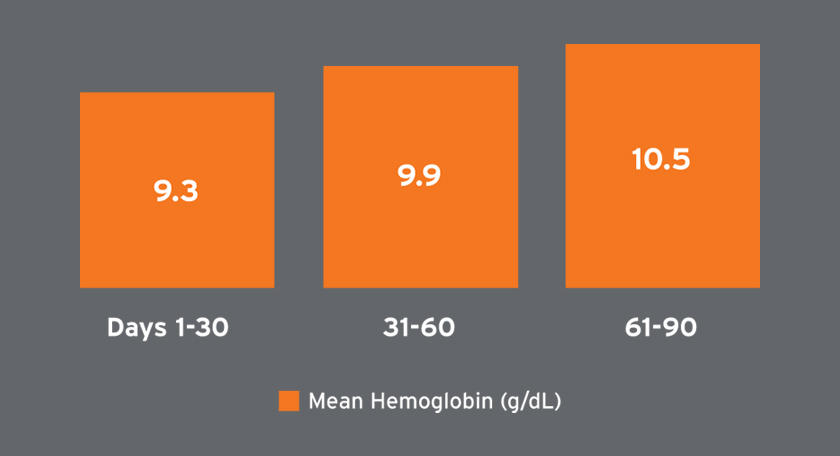
FIGURE 7 | Total Venofer dose and iron labs
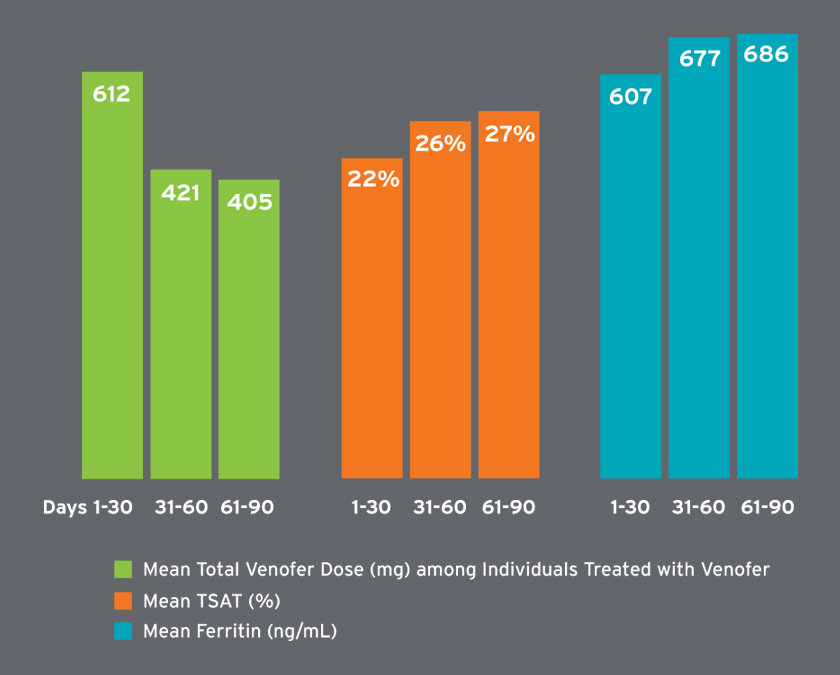
FIGURE 8 | Mircera dose per HD treatment
MEASURES OF RENAL FUNCTION AND VOLUME MANAGEMENT
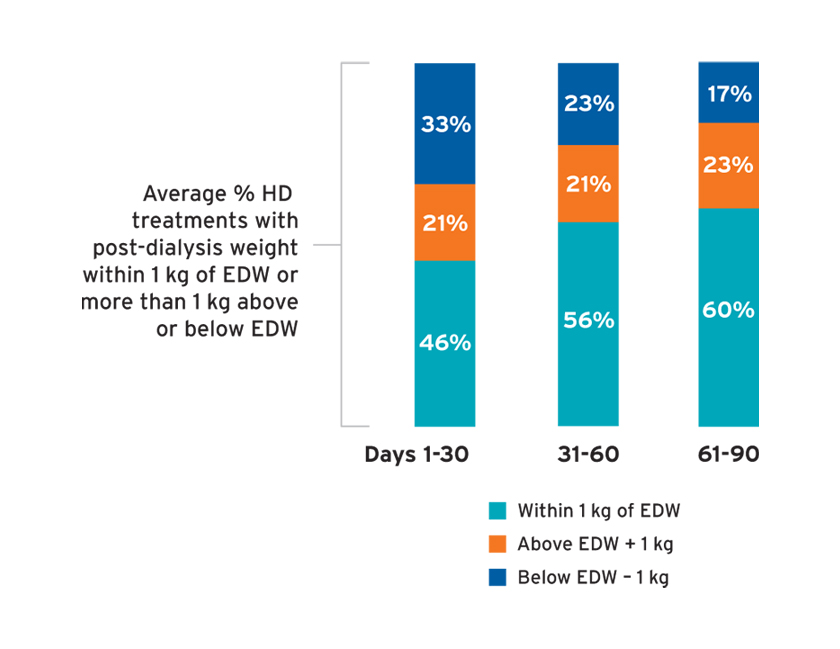
Among individuals with measures of 24-hour urine urea clearance, the median urine urea clearance was 4.8 to 5.3 mL/min (Figure 9), whereas the median serum creatinine ranged from 4.0 to 4.3 mg/dL. The median 24-hour urine volume (and number of individuals with urine volume measured) in days 1-30, 31-60, and 61-90 was 1.1 L (N=2,164); 1.0 L (N=1,315); and 1.0 L (N=574), respectively. In the first 30 days, less than 50 percent of treatments resulted in a post-dialysis weight within 1 kg of the estimated dry weight (EDW), and 33 percent of treatments resulted in post-dialysis weights more than 1 kg below the EDW (Figure 10). The average percent of treatments below EDW was lower over time (Figure 10).
FIGURE 9 | Residual kidney function
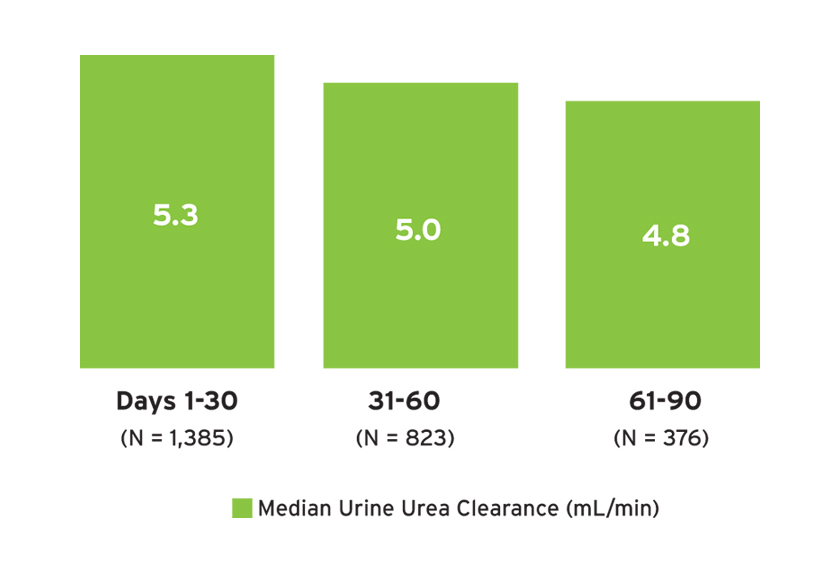
FIGURE 10 | Average percent of treatments with post-dialysis weight within 1 kg of estimated dry weight (EDW)
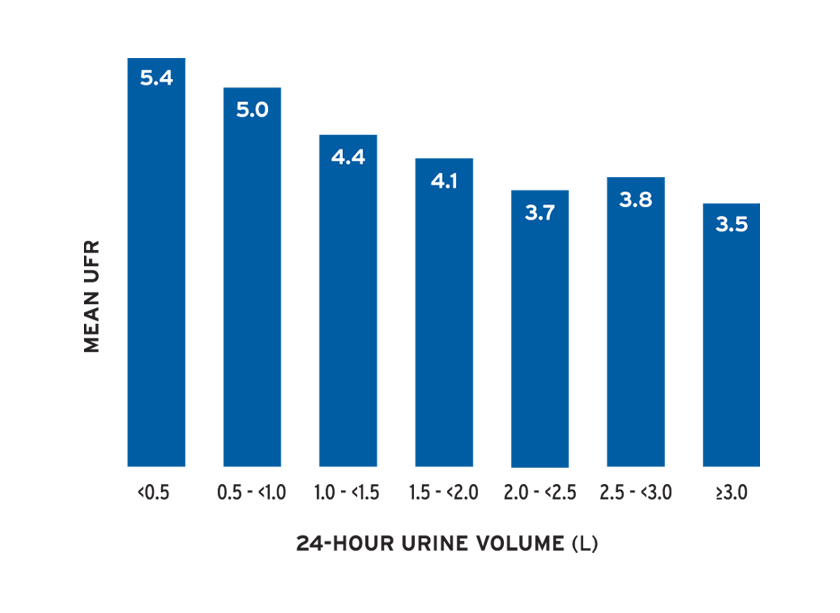
The mean ultrafiltration rate (UFR) normalized by post-treatment weight ranged from 4.9 to 5.9 mL/hr/kg (Figure 11a). The mean UFR differed by treatment time and by urine volume (Figure 11b).
FIGURE 11A | Ultrafiltration rate
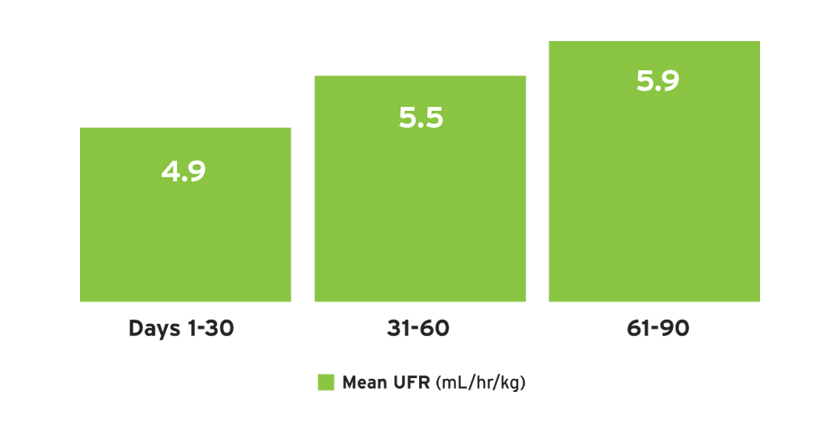
FIGURE 11B | UFR (mL/hr/kg) by urine volume in first 30 days
OUTCOMES
Individuals with AKI-D were followed for up to 150 days or until recovery of kidney function, transition to ESRD, withdrawal from dialysis, transfer to a non-Fresenius Kidney Care facility, loss to follow-up, death, or June 30, 2018. Cumulative 90- and 150-day outcomes are shown in Figure 12.
FIGURE 12 | AKI-D cumulative outcomes
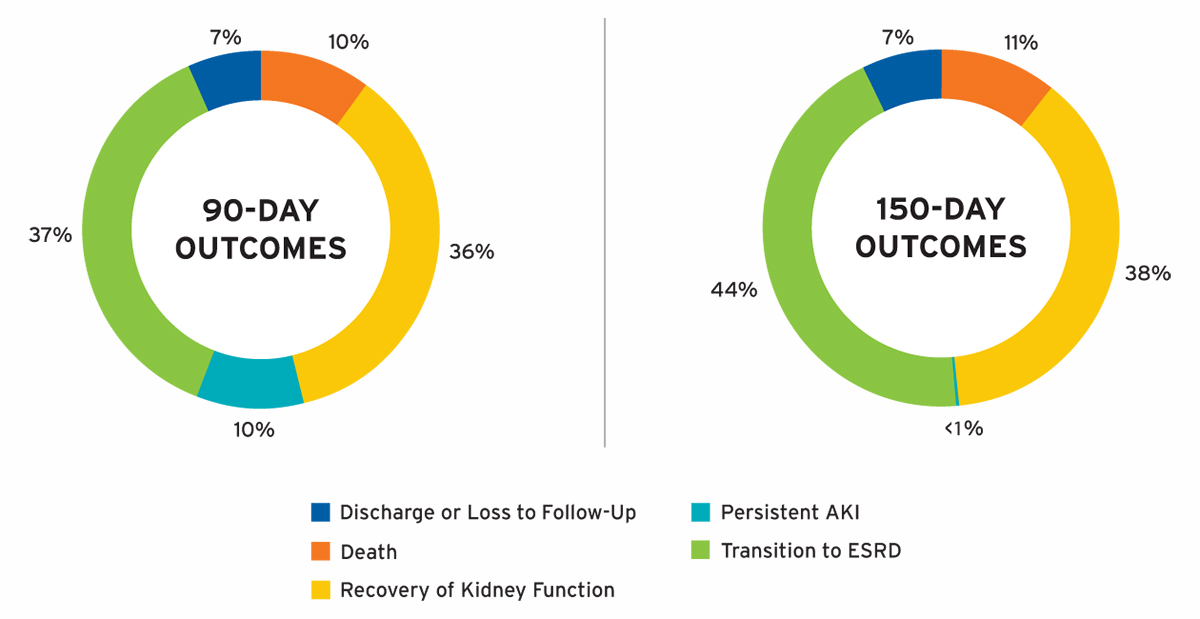
Outcomes at 90 days of AKI-D were notable for the following: 36 percent of patients recovered kidney function, 37 percent transitioned to ESRD, 10 percent died, and 10 percent had persistent AKI. Among individuals with sustained AKI for more than 90 days, 16 percent recovered kidney function by day 150. In the majority of individuals with AKI-D in whom dialysis was withdrawn, data was available on whether patients recovered kidney function or died within 30 days of withdrawal of dialysis, and these patients were classified as recovery of kidney function or death, respectively. Death was only ascertained in patients with a diagnosis of AKI-D and was not assessed after the transition to ESRD or recovery of kidney function; therefore, the findings may underestimate the overall 90-day mortality of patients initiating outpatient treatment for AKI-D.
DISCUSSION
In conclusion, approximately 20 percent of patients admitted to Fresenius Kidney Care in-center hemodialysis facilities for the initiation of dialysis had a diagnosis of AKI-D. Among patients with AKI-D, 36 percent recovered kidney function and 37 percent transitioned to ESRD in the first 90 days. Late recovery of kidney function was also observed among individuals with prolonged AKI-D. Prior studies of in-hospital AKI-D transitioned to outpatient dialysis management have been single-center studies with cohorts ranging from approximately 100 to 200 AKI patients.12,13,14,15 These studies found that 25 to 43 percent of patients with AKI-D recovered kidney function in the outpatient setting, and in the first 90 days, 45 to 49 percent transitioned to ESRD and 9 percent died.16,17,18
Similar to published studies, this analysis found a high proportion of patients recovered kidney function, and a comparable mortality rate of 10 percent was observed in the first 90 days. FMCNA's initial findings contribute to a broader understanding of outcomes in this population given the large and geographically diverse cohort examined post-implementation of the CMS policy.
Given the increasing incidence of AKI-D, decreasing mortality, variability in the need for dialysis following hospital discharge, and the changing care settings for AKI-D, estimating the number of individuals in the United States with AKI-D who will require outpatient dialysis support is challenging.19,20,21,22,23,24 Based on its experience, FMCNA estimates that more than 100,000 patients with AKI-D will be managed in an outpatient dialysis setting in the next five years in the United States. The changing care paradigm for AKI-D creates new opportunities to further study and understand sustained AKI-D, but significantly more research is needed to understand the optimal treatment strategies for patients with prolonged AKI-D.25,26
Importantly, the change in CMS policy has allowed more patients with AKI-D the opportunity to be treated outside of the hospital and receive dialysis support while recovering kidney function. In addition, it has presumably encouraged appropriate utilization of hospital resources—an anticipated benefit of the change in policy.27 With the experience gained over the past two years, there is an opportunity to evolve the care of AKI-D to continue to optimize outcomes in this medically complex population.
Meet Our Experts
LORIEN S. DALRYMPLE, MD, MPH
Vice President, Epidemiology and Research, Fresenius Medical Care
Board certified in nephrology, Lorien Dalrymple received her bachelor's degree from Duke University, her medical degree from the University of Colorado, and her Master of Public Health from the University of Washington. She is co-chair of the National Quality Forum Renal Standing Committee and co-chair of the KHI ESRD Global Data Standard Workgroup.
NORMA J. OFSTHUN, PhD
Senior Vice President, Data Governance, Fresenius Medical Care
Norma Ofsthun leads FMCNA's data governance efforts and chairs the company's Data Governance Program. She has collaborated with numerous Fresenius physicians and outside collaborators on a variety of research publications in the renal field. Over the past 20 years, Norma's team has provided data and reports for key clinical initiatives such as the conversion from reuse to single-use dialyzers, the development of electronic algorithms for drug dosing, and the current AKI program. Norma holds her doctorate in chemical engineering from the Massachusetts Institute of Technology and holds numerous patents related to membrane-based medical devices.
References
- Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol 2013;24(1):37-42.
- Wald R, McArthur E, Adhikari NK, et al. Changing incidence and outcomes following dialysis-requiring acute kidney injury among critically ill adults: population-based cohort study. Am J Kidney Dis 2015;65(6):870-77.
- Hsu et al. Temporal changes.
- The VA/NIH Acute Renal Failure Trial Network. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 2008 Jul;359(1):7-20.
- Mehta RL, Pascual MT, Soroko S, et al. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int 2004;66(4):1613-21.
- Cerda J, Liu KD, Cruz DN, et al. Promoting kidney function recovery in patients with AKI requiring RRT. Clin J Am Soc Nephrol 2015;10(10):1859-67.
- Fagugli RM, Patera F, Battistoni S, Tripepi G. Outcome in noncritically ill patients with acute kidney injury requiring dialysis: effects of differing medical staffs and organizations. Medicine (Baltimore) 2016;95(30):e4277.
- Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 2005;294(7):813-18.
- Heung M, Faubel S, Watnick S, et al. Outpatient dialysis for patients with AKI: a policy approach to improving care. Clin J Am Soc Nephrol 2015;10(10):1868-74.
- Ibid.
- Centers for Medicare and Medicaid Services. Acute kidney injury and ESRD facilities. Last updated April 3, 2018. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ESRDpayment/AKI-and-ESRD-Facilities.html.
- Gautam SC, Brooks CH, Balogun RA, Xin W, Ma JZ, Abdel-Rahman EM. Predictors and outcomes of post-hospitalization dialysis dependent acute kidney injury. Nephron 2015;131(3):185-90.
- Hickson LJ, Chaudhary S, Williams AW, et al. Predictors of outpatient kidney function recovery among patients who initiate hemodialysis in the hospital. Am J Kidney Dis 2015;65(4):592-602.
- Pajewski R, Gipson P, Heung M. Predictors of post-hospitalization recovery of renal function among patients with acute kidney injury requiring dialysis. Hemodial Int 2018;22(1):66-73.
- Rathore AS, Chopra T, Ma JZ, Xin W, Abdel-Rahman EM. Long-term outcomes and associated risk factors of post-hospitalization dialysis-dependent acute kidney injury patients. Nephron 2017;137(2):105-112.
- Gautam et al. Predictors and outcomes of post-hospitalization.
- Hickson et al. Predictors of outpatient kidney function recovery.
- Pajewski et al. Predictors of post-hospitalization recovery.
- Hsu et al. Temporal changes in incidence.
- Wald et al. Changing incidence and outcomes.
- Cerda et al. Promoting kidney function recovery in patients.
- Fagugli et al. Outcome in noncritically ill patients.
- Uchino et al. Acute renal failure in critically ill patients.
- Heung et al. Outpatient dialysis for patients with AKI.
- Cerda et al. Promoting kidney function recovery in patients.
- Heung et al. Outpatient dialysis for patients with AKI.
- Ibid.



