HOW THE “BUNDLE” HAS CHANGED CLINICAL CARE AT FRESENIUS KIDNEY CARE
In January 2011, a new reimbursement system was implemented for Medicare beneficiaries with ESRD. Prior to that, the Centers for Medicare and Medicaid Services (CMS) paid providers using a combination of composite rate (a single reimbursement for all technical, personnel, and operational expenses) and a separate payment for pharmaceuticals administered during the dialysis treatment. The “Bundle” in relation to Medicare reimbursements for dialysis treatments refers to a single payment to combine both the technical, personnel, operational expenses and the pharmaceuticals admistered during dialysis treatment . In addition to this base rate, modifications to payments were made based on demographic characteristics of patients, the presence of certain comorbid conditions, and the facility’s performance on a series of quality of care metrics called the Medicare Quality Improvement Program (QIP).
Improving Efficient Use of Resources
The move to prospective payment created the impetus to define processes focused on more efficient use of resources, particularly the medications given as part of the dialysis treatment. The challenge for Fresenius Kidney Care (FKC) was to achieve these medication efficiencies while continuing to maintain or improve quality outcomes. Focus was applied primarily on two areas: use of erythropoiesis-stimulating agents (ESAs) to correct the anemia of chronic kidney disease, and vitamin D treatment of bone and mineral metabolism abnormalities. Organizing clear processes to assess performance of algorithms has led to decreased variability in results.
ESAs
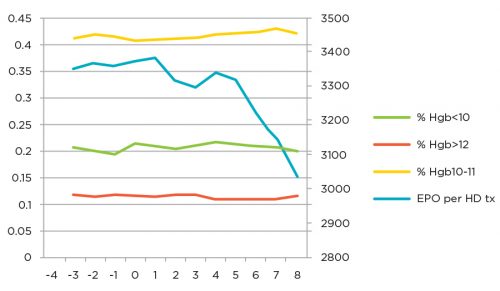
Initially, efforts were directed at optimizing use of the predominant ESA, Epogen, within the clinics. Processes included evaluation and improvement of the Anemia Treatment Algorithm and a focus on patients using high doses of ESA. Collaboration with the Renal Research Institute allowed the testing of different decision points within the algorithm and resulted in modification of that care path. A group of centrally based corporate anemia managers were enlisted to review situations where high doses were being used and provide recommendations to physicians to optimize care. When operationalized, these projects resulted in a reduction of the mean dose of Epogen used per hemodialysis treatment from 3,350 units to 3,050 units, while hemoglobin outcomes were maintained or improved (see Figure 1).
Figure 1 | Monthly performance (at 8 months)
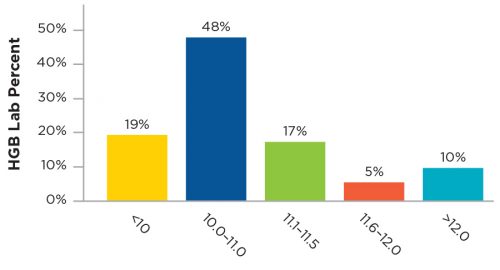
The market entry of Mircera (PEGylated epoetin beta) by Roche, created two opportunities. On a financial level, it created a competitive pricing environment where previously only a single manufacturer’s product was available. From a clinical standpoint, Mircera presented the chance to gain experience with a truly long-acting ESA. The project that was launched to introduce Mircera became a valuable learning exercise in cross-functional cooperation. Every part of FMCNA—Medical Office, Clinical Services, Operations, Value Based Services, Spectra Lab, supply chain, financial, and education—came together to introduce this new medication and hone operational and clinical procedures. Over 24 months, Mircera reached 130,000 patients and provided both outstanding clinic results and significant financial efficiency (Figure 2).
Figure 2 | Hemoglobin Results of Mircera Dosing
Vitamin D
Intravenous vitamin D analogs have been the mainstay of treatment of the bone and mineral complications of ESRD for many years. Historically, an oral version of vitamin D had been used but fell out of favor as the more expensive parenteral versions became preferred. After an extensive literature review, a treatment algorithm using oral calcitriol was developed, along with a process for monitoring safety and efficacy of this treatment. Through detailed communication of data to the medical staff, ultimately 60 percent of vitamin D usage was transitioned to oral calcitriol. While significant savings were realized, levels of the relevant metabolites (parathyroid hormone, calcium, and phosphorus) were controlled to levels equal to those achieved with intravenous vitamin D. Significantly, patient survival and hospitalization rates were equal to or better than those achieved before the introduction of oral calcitriol (Figures 3 and 4).
Figure 3 | Multivariable Cox proportional-hazards models examining hazard ratios associated with oral Calcitrol treatment as compared with IV vitamin D treatment.
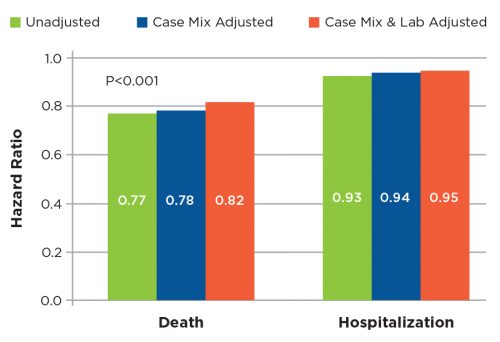
Figure 4 | Kaplan-Meier survival curves for mortality and hospitalization one-year follow up
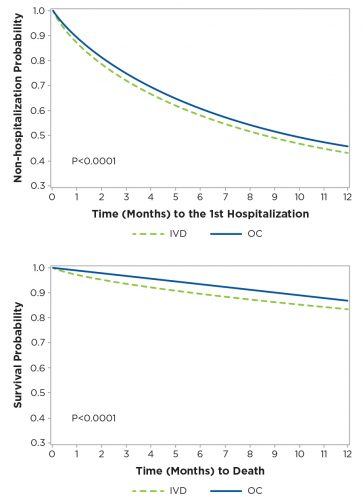
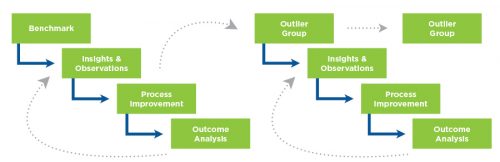
Figure 5 | Recursive quality management process focus on outlier patient group
Moving the Needle Through Quality Management
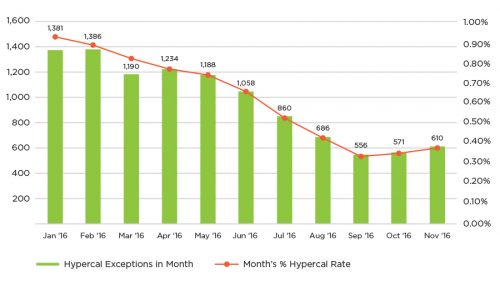
The QIP program examines a variety of metrics that are felt to be indicative of good dialysis care. For two of them, dialysis adequacy (as measured by single pool Kt/V) and hypercalcemia above 10.2 mg/dL, it was particularly difficult to demonstrate improvement because achievement levels were already quite high. In order to “move the needle” on these areas, it was necessary to develop an approach that focused on the relatively small number of patients who failed to meet these criteria. A reassessment of continuous quality improvement (CQI) activity was undertaken (Figure 5).
To achieve improvement, special emphasis was placed on the recursive process of outcome analysis, planning an intervention, evaluation of the impact of the change, and repetition of the CQI cycle with focus on the “outliers” who had not improved.
This technique of “outlier management” was adopted as a fundamental activity of the FKC Quality department, with regional quality managers becoming experts in root cause analysis and application of corrective actions. Over the course of 2016, fewer patients entered this outlier status, and the performance level of the company improved (Figures 6 and 7).
This approach to quality improvement is now being applied to other clinical areas including volume management and elimination of venous dialysis catheters.
Figure 6 | 2016 CQI Kt/V results
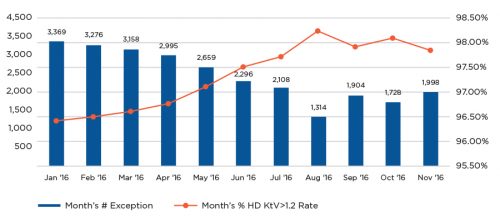
Figure 7 | 2016 CQI hypercalcemia results
Conclusion
The introduction of the “Bundle” provided a stimulus to change clinical algorithms and quality improvement activities to enhance patient outcomes while delivering better economic results. The activities necessary to achieve these efficiencies crossed all segments of the FMCNA organization, including Medical Office, Clinical Services, Finance, Data Analytics, Compliance, and Legal participation.
Meet the Expert
Jeffrey Hymes, MD
Senior Vice President and Chief Medical Officer, Fresenius Kidney Care
Chairman, Pharmacy & Therapeutics Committee
Dr. Jeffrey Hymes is Senior Vice President and Chief Medical Officer for Fresenius Kidney Care and Chairman of its Pharmacy and Therapeutics Committee. He is a graduate of Yale College and the Albert Einstein College of Medicine and served his medical internship and residency at Yale New Haven Medical Center and received subspecialty training in nephrology at Boston University. Dr. Hymes is board certified in internal medicine, nephrology and critical care. He was a co-founder of REN Corporation in 1986 and National Nephrology Associates (NNA) in 1998. Dr. Hymes served as NNA’s President and Chief Medical Officer from 1998 to 2004. He was President of Nephrology Associates, a 32-physician nephrology practice serving Middle Tennessee. From 1989 to 2012, he was President of Nephrology Associates, a 32-physician nephrology practice serving Tennessee, and is a former member of the Renal Physician Association’s Board of Directors.



